
Environmental Management

What We Offer
remediation and implementation of environmental management systems
= acc. to EN ISO 14001
2 Interim Environmental Manager responsible for implementation, maintenance, and monitoring of required processes
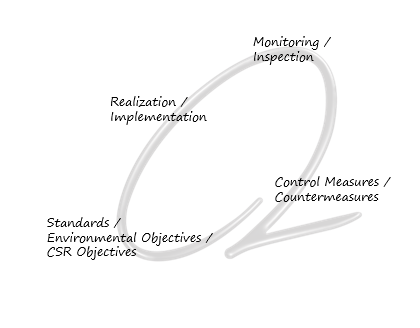
2 Preparation and implementation of environmental targest
2 Installation of an integrated management system for process-oriented control or relevant objectives
7
The Green Management Loops
...
Our Services
Just Blogging

Using an environmental management system generates significantly added value in case legal applicable safety at work requirements are defined as quality objectives...
NEWS


