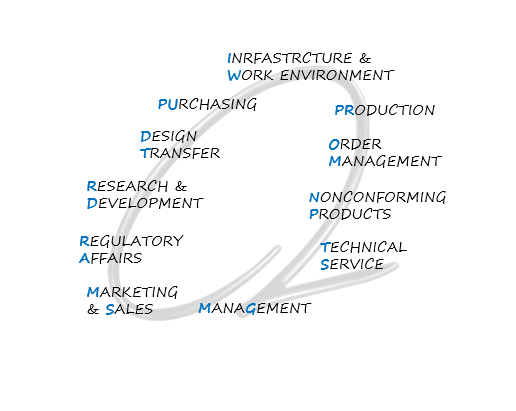
The QC Loop Concept
We provide full support and services for the identification of applicable standards and full compliance with self-defined and regualtory requriements.
We develop customer-tailored process loops for enhanced management controls, efficiency and the generation of required proofs of compliance to be prepared for regulatory challenges.
Implementation of regulatory control concepts are the basis for measurable solutions. We design and implement integrated management systems to control all kind of requirements on quality, environment, and safety due to lean and flexible processes.
7
Quality Control Loop
We focus on cooperative teamwork and hybrid consulting with fading borders between consultancy and practical implementation. Our interim managers are fit for service from day one and manage complex remediation projects as well as the daily business.
Just Blogging

Using an environmental management system generates significantly added value in case legal applicable safety at work requirements are defined as quality objectives...



